- cross-posted to:
- medicine@mander.xyz
- cross-posted to:
- medicine@mander.xyz
The study also found that the virus can survive and grow inside the cells that form plaque—the buildup of fat-filled cells that narrow and stiffen the arteries leading to atherosclerosis. If the plaque breaks, it can block blood flow and cause a heart attack or a stroke. The SARS-CoV-2 infection makes the situation worse by inflaming the plaque and increasing the chance that it breaks free.
![]()
A recent study of more than 800,000 people led by Fabio Angeli, a cardiologist at University of Insubria in Varese, Italy, has shown that COVID-19 patients develop high blood pressure twice as often as others. More worrying is that the risk of cardiac diseases can also rise for patients who suffered only mild COVID symptoms.
A virus that can cause autoimmune issues and heart disease, and is mutating several times faster than other respiratory viruses, and our plan is to infect everyone as much as possible to "build immunity".
From what I can find, most data about the amount of heart disease and strokes hasn't been updated since before the pandemic. It would be nice if that were available to see how much of an impact covid is having.
This article is paywalled, so the full article is in the spoiler tags
full article
Now we know how COVID attacks your heart Even patients with mild COVID symptoms could face a higher risk of developing heart disease and stroke
By Sanjay Mishra
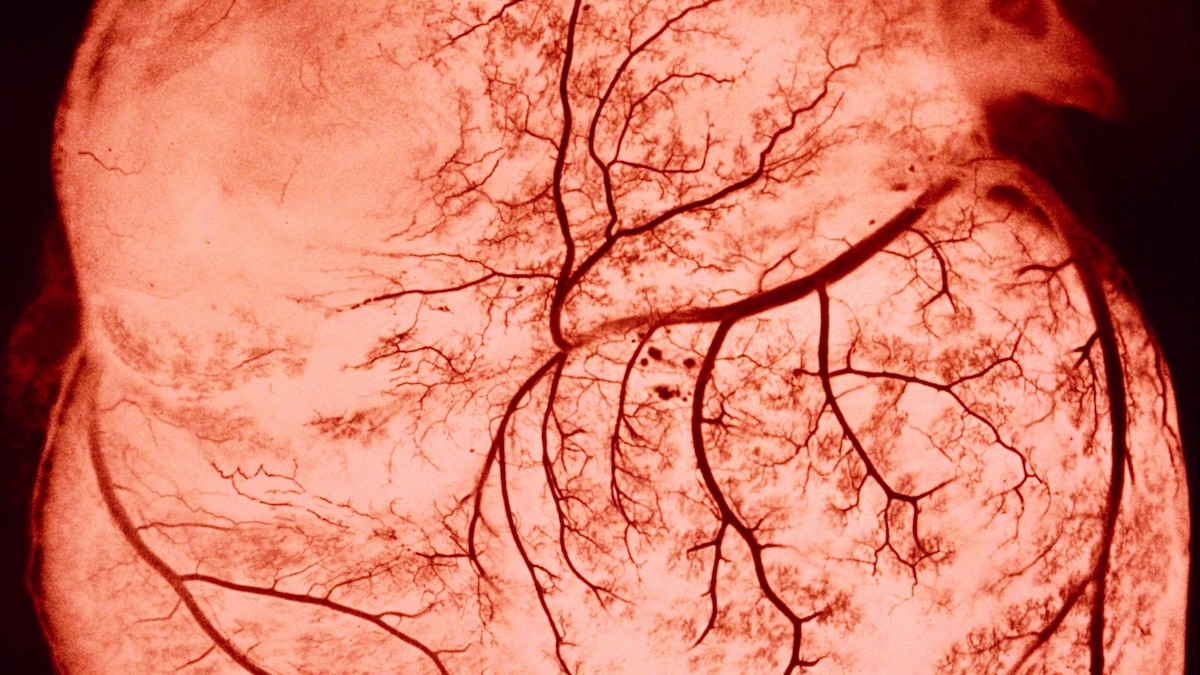
Nov 07, 2023 Scientists have noticed that COVID-19 can trigger serious cardiovascular problems, especially among older people who have a buildup of fatty material in their blood vessels. But now a new study has revealed why and shown that SARS-CoV-2, the virus that causes COVID-19, directly infects the arteries of the heart.
The study also found that the virus can survive and grow inside the cells that form plaque—the buildup of fat-filled cells that narrow and stiffen the arteries leading to atherosclerosis. If the plaque breaks, it can block blood flow and cause a heart attack or a stroke. The SARS-CoV-2 infection makes the situation worse by inflaming the plaque and increasing the chance that it breaks free.
This can explain long-term cardiovascular effects seen in some, if not all, COVID-19 patients.
SARS-CoV-2 virus has already been found to infect many organs outside the respiratory system. But until now it hadn't been shown to attack the arteries.
"No one was really looking if there was a direct effect of the virus on the arterial wall," says Chiara Giannarelli, a cardiologist at NYU Langone Health, in New York, who led the study. Giannarelli noted that her team detected viral RNA—the genetic material in the virus—in the coronary arteries. “You would not expect to see [this] several months after recovering from COVID.”
Mounting evidence now shows that SARS-CoV-2 is not only a respiratory virus, but it can also affect the heart and many other organ systems, says Ziyad Al-Aly, a clinical epidemiologist at Washington University in St. Louis. Al-Aly's research has shown that the risk of developing heart and cardiovascular diseases, including heart failure, stroke, irregular heart rhythms, cardiac arrest, and blood clots increases two to five times within a year of COVID-19, even when the person wasn't hospitalized.
"This important study links, for the first time, directly the SARS-CoV-2 virus with atherosclerotic plaque inflammation," says Charalambos Antoniades, chair of cardiovascular medicine at the University of Oxford, United Kingdom.
Virus triggers the inflammation in plaque
A recent study of more than 800,000 people led by Fabio Angeli, a cardiologist at University of Insubria in Varese, Italy, has shown that COVID-19 patients develop high blood pressure twice as often as others. More worrying is that the risk of cardiac diseases can also rise for patients who suffered only mild COVID symptoms.
"I saw a patient who now has a defibrillator, and she didn't even have a severe [COVID] illness," says Bernard Gersh, a cardiologist at Mayo Clinic, Rochester, Minnesota.
Wondering whether the cardiovascular damage during COVID was due to the virus directly attacking the blood vessels, the NYU team analyzed autopsied tissue from the coronary arteries and plaque of older people who had died from COVID-19. They found the virus was present in the arteries regardless of whether the fatty plaques were big or small.
"The original finding in this study is that the virus was convincingly found in the plaque in the coronary artery," says Juan Carlos Kaski, a cardiovascular specialist at St George's, University of London, who was not involved in the study.
The NYU team found that in the arteries, the virus predominantly colonized the white blood cells called macrophages. Macrophages are immune cells that are mobilized to fight off an infection, but these same cells also absorb excess fats—including cholesterol from blood. When microphages load too much fat, they change into foam cells, which can increase plaque formation.
To confirm that the virus was indeed infecting and growing in the cells of the blood vessels, scientists obtained arterial and plaque cells—including macrophages and foam cells—from healthy volunteers. Then they grew these cells in the lab in petri dishes and infected them with SARS-CoV-2.
Giannarelli found that although virus infected macrophages at a higher rate than other arterial cells, it did not replicate in them to form new infectious particles. But when the macrophages had become loaded with cholesterol and transformed into foam cells, the virus could grow, replicate, and survive longer.
"We found that the virus tended to persist longer in foam cells," says Giannarelli. That suggests that foam cells might act as a reservoir of SARS-CoV-2. Since more fatty buildup would mean a greater number of foam cells, plaque can increase the persistence of the virus or the severity of COVID-19.
Scientists found that when macrophages and foam cells were infected with SARS-CoV-2 they released a surge of small proteins known as cytokines, which signal the immune system to mount a response against a bacterial or viral infection. In arteries, however, cytokines boost inflammation and formation of even more plaque.
"We saw that there was a degree of inflammation [caused] by the virus that could aggravate atherosclerosis and cardiovascular events," says Giannarelli.
These findings also confirm previous reports that measuring inflammation in the blood vessel wall can diagnose the extent of long-term cardiovascular complications after COVID-19, says Antoniades.
"What this study has found is that plaque rupture can be accelerated and magnified by the presence of the virus," says Kaski.
Understanding heart diseases after COVID
While this new research clearly shows that SARS-CoV-2 can infect, grow, and persist in the macrophages of plaques and arterial cells, more studies are needed to fully understand the many ways COVID-19 can alter cardiac health.
"The NYU study identifies one potential mechanism, especially the viral reservoir, to explain the possible effects" says Gersh. "But It's not going to be the only mechanism."
This study only analyzed 27 samples from eight elderly deceased patients, all of whom already had coronary artery disease and were infected with the original strains of virus. So, the results of this study do not necessarily apply to younger people without coronary artery disease; or to new variants of the virus, which cause somewhat milder disease, says Angeli.
"We do not know if this will happen in people who have been vaccinated," says Kaski. "There are lots of unknowns."
It is also not clear whether and to what extent the high inflammatory reaction observed in the arteries of patients within six months after the infection, as shown in the new study, will last long-enough to trigger new plaque formation. "New studies are needed to show the time-course of the resolution of vascular inflammation after the infection," says Antoniades.
COVID patients should watch for any new incidence of shortness of breath with exertion, chest discomfort, usually with exertion, palpitations, loss of consciousness; and talk to their physician about possible heart disease.
Welp, guess I won't have to worry too much about saving enough to retire.

I don't think I've gotten COVID yet, but with all the mild and asymptomatic cases who knows at this point.
I'm in the exact situation. It's infuriating how the capitalists have just let this become so normalized.
Yeah, I'm extremely in the "is this long COVID or am I just getting old?" boat right now and it's frustrating to say the least.
Absolutely understand that feeling. I chalk it up to aging and stress with a slight possibility of long COVID on top of that. Just a big old stew of things that will melt your brain.
Pseudo-ML tailist bugboys calling everyone on the site idealist for saying we should mitigate COVID with the only tools we have left rather than being dying hog consumers on the trough that a dying imperial core accepts as normal.
edit: still mad over that thread. Most of them are still on the site too.
That thread was so upsetting. I made a follow-up thread on another account and got even more of that bullshit.
Once again, it's not a respiratory disease, it's an endothelial disease
The government has abandoned us when it comes to public health. We've got to take care of ourselves and each other now. We've got to do what we can--wear a mask, a respirator if you can, filter your air, ventilate, avoid crowded places, use a nasal spray, vaccinate-- do as many of these as you can. Heart disease is something you want to avoid getting if you can. Please, protect yourselves and your loved ones. Covid is spread in the air and you can reduce your chances of getting it if you filter and ventilate your air and avoid sharing air with people when possible. Don't give up. Each new infection carries the risk of permanent complications and it's important to keep protecting your loved ones community. Every time you protect yourself you protect all of us. Even preventing one infection matters.
COVID patients should watch for any new incidence of shortness of breath with exertion, chest discomfort, usually with exertion, palpitations, loss of consciousness; and talk to their physician about possible heart disease.
Show Show
Show
This could have been handled so easily and it would've been cheaper in the long run.
I feel so much anger and grief and being in an indifferent workplace makes it so much worse.
The build immunity plan will probably work, generationally, as everyone who has genes that make them susceptible to it will die more than people without.
So capitalists COULD solve this with medicine and societal changes but they'll just opt to "solve" it by allowing COVID to be a human evolutionary filter event instead. Disgusting
I don't think they even give a fuck about building immunity. They have their rich people meds, their rich people antivirals, their rich people testing, all the rich people state of the art healthcare. Why would they care if we die? We die all the time from preventable things like cancer, war, environmental poisoning, etc. All they care about is harvesting all the value and labor they can before we die.
Much like the rest of capitalism, I'm sure it'll be a Great Filter.
There will for sure be new surges of auto-immune diseases for future generations to deal with because of it too. Like with black death and Crohn's disease.
Article with links
Scientists have noticed that COVID-19 can trigger serious cardiovascular problems, especially among older people who have a buildup of fatty material in their blood vessels. But now a new study has revealed why and shown that SARS-CoV-2, the virus that causes COVID-19, directly infects the arteries of the heart.
The study also found that the virus can survive and grow inside the cells that form plaque—the buildup of fat-filled cells that narrow and stiffen the arteries leading to atherosclerosis. If the plaque breaks, it can block blood flow and cause a heart attack or a stroke. The SARS-CoV-2 infection makes the situation worse by inflaming the plaque and increasing the chance that it breaks free.
This can explain long-term cardiovascular effects seen in some, if not all, COVID-19 patients.
SARS-CoV-2 virus has already been found to infect many organs outside the respiratory system. But until now it hadn't been shown to attack the arteries.
"No one was really looking if there was a direct effect of the virus on the arterial wall," says Chiara Giannarelli, a cardiologist at NYU Langone Health, in New York, who led the study. Giannarelli noted that her team detected viral RNA—the genetic material in the virus—in the coronary arteries. “You would not expect to see [this] several months after recovering from COVID.”
Mounting evidence now shows that SARS-CoV-2 is not only a respiratory virus, but it can also affect the heart and many other organ systems, says Ziyad Al-Aly, a clinical epidemiologist at Washington University in St. Louis. Al-Aly's research has shown that the risk of developing heart and cardiovascular diseases, including heart failure, stroke, irregular heart rhythms, cardiac arrest, and blood clots increases two to five times within a year of COVID-19, even when the person wasn't hospitalized.
"This important study links, for the first time, directly the SARS-CoV-2 virus with atherosclerotic plaque inflammation," says Charalambos Antoniades, chair of cardiovascular medicine at the University of Oxford, United Kingdom.
Virus triggers the inflammation in plaque
A recent study of more than 800,000 people led by Fabio Angeli, a cardiologist at University of Insubria in Varese, Italy, has shown that COVID-19 patients develop high blood pressure twice as often as others. More worrying is that the risk of cardiac diseases can also rise for patients who suffered only mild COVID symptoms.
"I saw a patient who now has a defibrillator, and she didn't even have a severe [COVID] illness," says Bernard Gersh, a cardiologist at Mayo Clinic, Rochester, Minnesota.
Wondering whether the cardiovascular damage during COVID was due to the virus directly attacking the blood vessels, the NYU team analyzed autopsied tissue from the coronary arteries and plaque of older people who had died from COVID-19. They found the virus was present in the arteries regardless of whether the fatty plaques were big or small.
"The original finding in this study is that the virus was convincingly found in the plaque in the coronary artery," says Juan Carlos Kaski, a cardiovascular specialist at St George's, University of London, who was not involved in the study.
The NYU team found that in the arteries, the virus predominantly colonized the white blood cells called macrophages. Macrophages are immune cells that are mobilized to fight off an infection, but these same cells also absorb excess fats—including cholesterol from blood. When microphages load too much fat, they change into foam cells, which can increase plaque formation.
To confirm that the virus was indeed infecting and growing in the cells of the blood vessels, scientists obtained arterial and plaque cells—including macrophages and foam cells—from healthy volunteers. Then they grew these cells in the lab in petri dishes and infected them with SARS-CoV-2.
Giannarelli found that although virus infected macrophages at a higher rate than other arterial cells, it did not replicate in them to form new infectious particles. But when the macrophages had become loaded with cholesterol and transformed into foam cells, the virus could grow, replicate, and survive longer.
"We found that the virus tended to persist longer in foam cells," says Giannarelli. That suggests that foam cells might act as a reservoir of SARS-CoV-2. Since more fatty buildup would mean a greater number of foam cells, plaque can increase the persistence of the virus or the severity of COVID-19.
Scientists found that when macrophages and foam cells were infected with SARS-CoV-2 they released a surge of small proteins known as cytokines, which signal the immune system to mount a response against a bacterial or viral infection. In arteries, however, cytokines boost inflammation and formation of even more plaque.
"We saw that there was a degree of inflammation [caused] by the virus that could aggravate atherosclerosis and cardiovascular events," says Giannarelli.
These findings also confirm previous reports that measuring inflammation in the blood vessel wall can diagnose the extent of long-term cardiovascular complications after COVID-19, says Antoniades.
"What this study has found is that plaque rupture can be accelerated and magnified by the presence of the virus," says Kaski.
Understanding heart diseases after COVID
While this new research clearly shows that SARS-CoV-2 can infect, grow, and persist in the macrophages of plaques and arterial cells, more studies are needed to fully understand the many ways COVID-19 can alter cardiac health.
"The NYU study identifies one potential mechanism, especially the viral reservoir, to explain the possible effects" says Gersh. "But It's not going to be the only mechanism."
This study only analyzed 27 samples from eight elderly deceased patients, all of whom already had coronary artery disease and were infected with the original strains of virus. So, the results of this study do not necessarily apply to younger people without coronary artery disease; or to new variants of the virus, which cause somewhat milder disease, says Angeli.
"We do not know if this will happen in people who have been vaccinated," says Kaski. "There are lots of unknowns."
It is also not clear whether and to what extent the high inflammatory reaction observed in the arteries of patients within six months after the infection, as shown in the new study, will last long-enough to trigger new plaque formation. "New studies are needed to show the time-course of the resolution of vascular inflammation after the infection," says Antoniades.
COVID patients should watch for any new incidence of shortness of breath with exertion, chest discomfort, usually with exertion, palpitations, loss of consciousness; and talk to their physician about possible heart disease.
Well, I wasn't planning on making it past 60 anyway and this just adds to those odds.